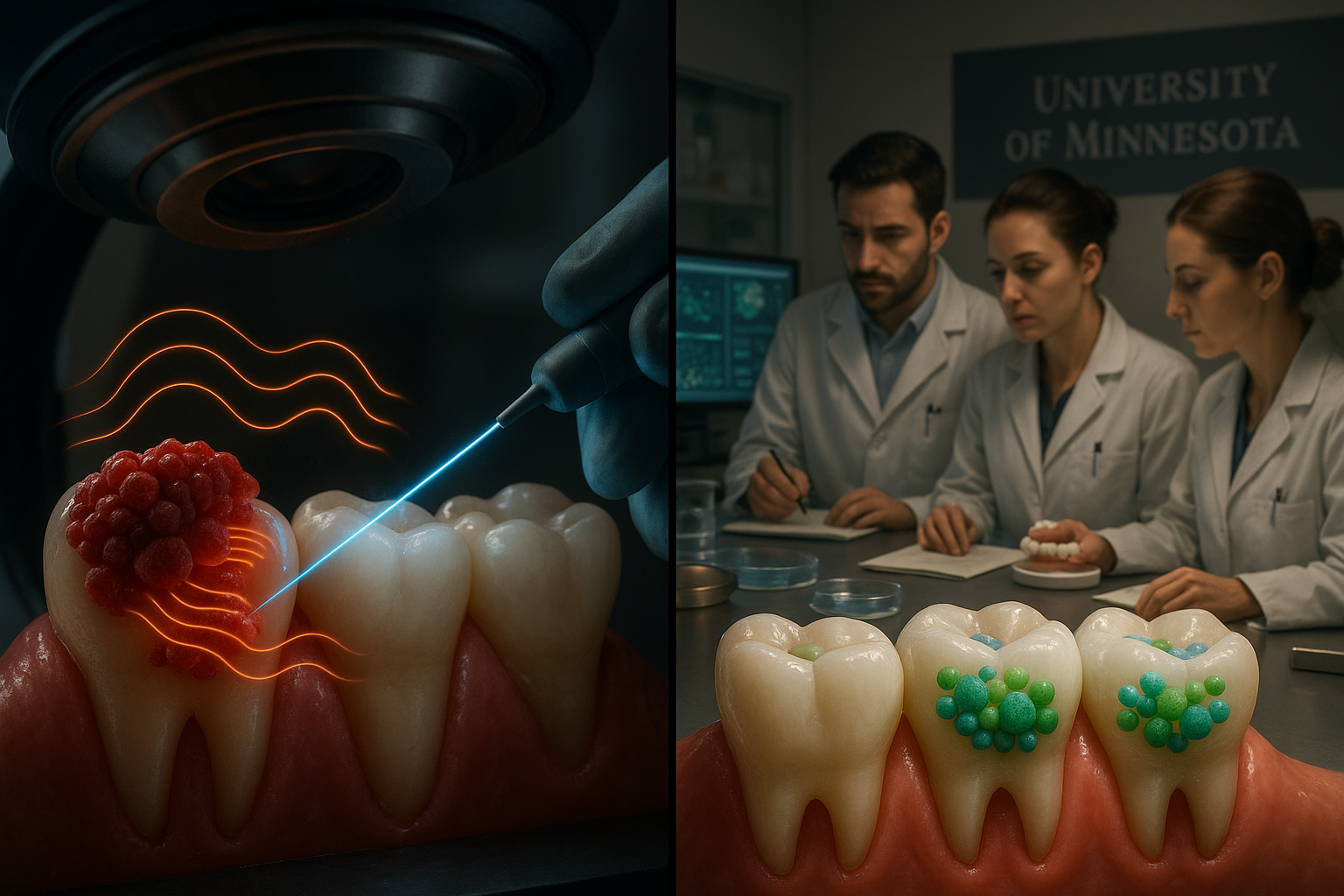
Scientists at the University of Minnesota have shown that disrupting how oral bacteria communicate can shift dental plaque toward communities associated with better oral health, potentially opening the door to new ways of preventing gum disease without wiping out beneficial microbes.
Bacteria in the human mouth use a process called quorum sensing to coordinate their behavior through chemical signals, including molecules known as N-acyl homoserine lactones (AHLs). Inside the mouth, roughly 700 bacterial species inhabit different surfaces and can exchange information and act collectively.
Researchers from the University of Minnesota's College of Biological Sciences and School of Dentistry set out to determine whether interfering with this communication could help prevent plaque buildup and promote a healthier oral environment, according to a summary from the university and ScienceDaily.
The study, published in late 2025 in the journal npj Biofilms and Microbiomes, found that bacteria in dental plaque produce AHL signals in oxygen-rich areas above the gumline, and that these messages can be detected by bacteria living in oxygen-poor regions beneath the gumline. When the researchers used specialized enzymes called lactonases to remove AHL signals in plaque-derived communities grown under 5% CO₂ (aerobic-like conditions), they observed an enrichment of commensal and pioneer colonizers that are associated with oral health. Under anaerobic conditions, adding AHLs instead promoted the growth of late colonizers that are often linked to disease.
"Dental plaque develops in a sequence, much like a forest ecosystem," said Mikael Elias, an associate professor at the University of Minnesota and senior author of the study. "Pioneer species like Streptococcus and Actinomyces are the initial settlers in simple communities—they're generally harmless and associated with good oral health. Increasingly diverse late colonizers include the 'red complex' bacteria like Porphyromonas gingivalis, which are strongly linked to periodontal disease. By disrupting the chemical signals bacteria use to communicate, one could manipulate the plaque community to remain or return to its health-associated stage."
Lead author Rakesh Sikdar emphasized how much oxygen conditions influenced the results. "What's particularly striking is how oxygen availability changes everything," Sikdar said. "When we blocked AHL signaling in aerobic conditions, we saw more health-associated bacteria. But when we added AHLs under anaerobic conditions, we promoted the growth of disease-associated late colonizers. Quorum sensing may play very different roles above and below the gumline, which has major implications for how we approach treatment of periodontal diseases."
The findings suggest that carefully targeting bacterial communication—rather than killing bacteria outright—could become a strategy for managing oral biofilms and reducing the risk of periodontal disease by maintaining a balanced microbiome. According to the University of Minnesota, the team plans to investigate how bacterial signaling varies across different regions of the mouth and at different stages of periodontal disease. They also note that similar quorum-sensing–based approaches could eventually inform microbiome-focused therapies in other parts of the body where microbial imbalances have been linked to illness, including certain cancers.
The research was funded by grants from the National Institutes of Health.