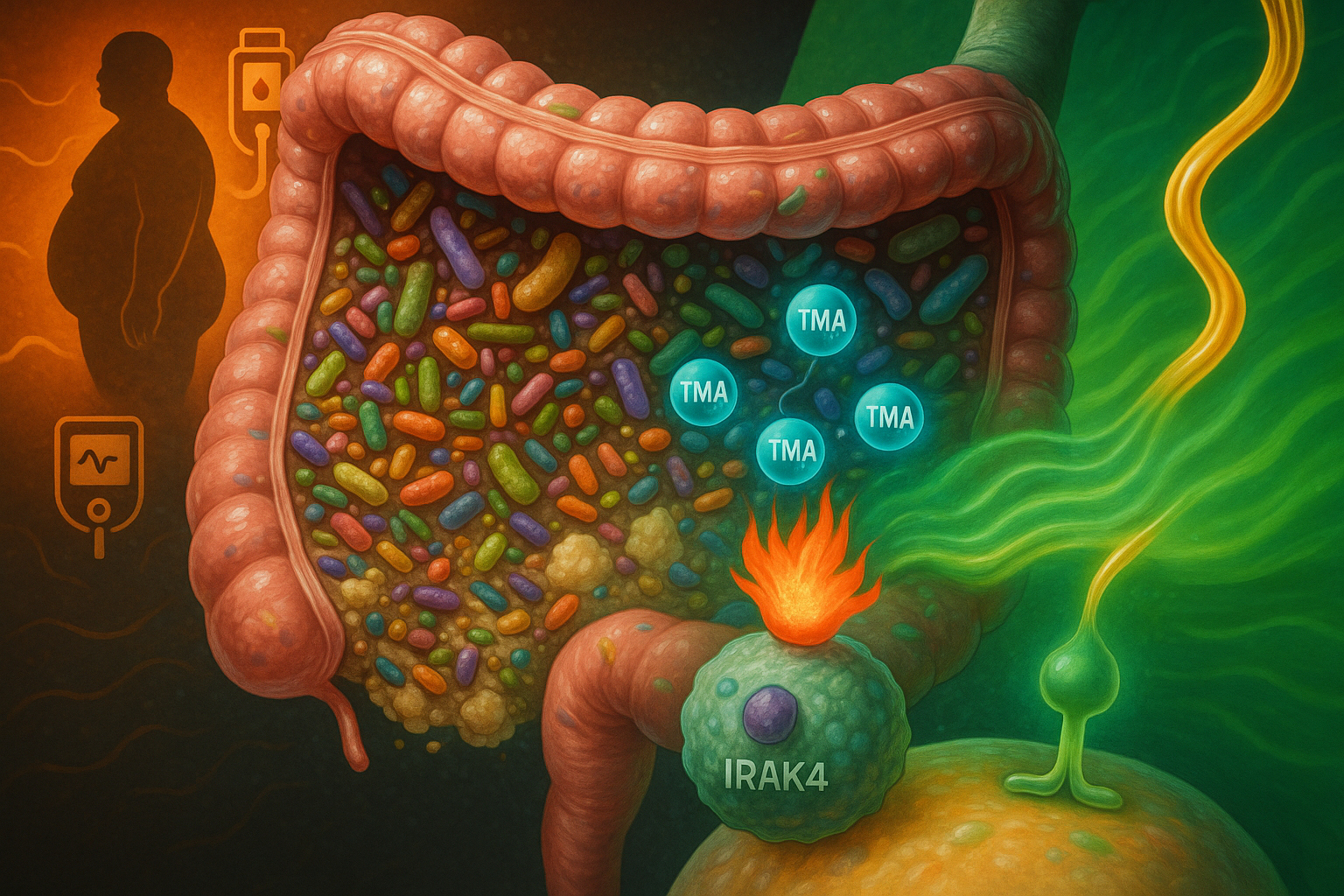
An international team of researchers has identified trimethylamine (TMA), a gut microbe metabolite produced from dietary nutrients such as choline, as a compound that inhibits the immune-signalling protein IRAK4, dampening inflammation and improving insulin action in experimental models. The discovery, reported in Nature Metabolism, suggests a potential new way to counter some of the harmful metabolic effects of high-fat diets and opens avenues for future type 2 diabetes therapies, a disease affecting more than 500 million people worldwide.
An international study led by scientists at Imperial College London, the French National Centre for Scientific Research (CNRS), Université catholique de Louvain, INSERM in Paris and the University of Ottawa Heart Institute builds on years of research into how diet and the gut microbiome influence metabolism.
According to background described by the team and earlier work from co-author Professor Patrice Cani, high-fat diets can allow bacterial components such as lipopolysaccharides to enter the bloodstream, activating immune pathways and promoting the low-grade inflammation that contributes to insulin resistance in type 2 diabetes. That concept, sometimes referred to as "metabolic endotoxemia", was considered controversial when first proposed in the mid‑2000s but is now widely supported in the metabolic disease field.
In the new work, published 8 December 2025 in Nature Metabolism, researchers report that TMA, a small molecule generated by gut bacteria when they break down nutrients including choline in food, can modulate this inflammatory process.
The study shows that under a high‑fat diet, the signalling protein IRAK4 (interleukin‑1 receptor‑associated kinase 4) is a central regulator of immune activation that drives chronic, diet‑induced inflammation and impaired insulin responses. Using a combination of primary human cell models, mouse experiments and molecular screening approaches, the team found that TMA binds to IRAK4 and inhibits its kinase activity. In these experimental systems, TMA reduced inflammation linked to high‑fat feeding and improved glycaemic control and insulin sensitivity.
The researchers also report that TMA improved survival in mice exposed to lipopolysaccharide‑induced septic shock by attenuating overwhelming inflammatory responses, an effect consistent with its IRAK4‑blocking action. Genetic deletion or pharmacological inhibition of IRAK4 produced comparable improvements in metabolic and immune parameters in high‑fat‑fed mice, reinforcing IRAK4 as a potential drug target, according to the study in Nature Metabolism.
“This flips the narrative,” said Professor Marc‑Emmanuel Dumas of Imperial College London and CNRS, one of the senior authors, in a statement released by the University of Ottawa Heart Institute and other institutional partners. “We’ve shown that a molecule from our gut microbes can actually protect against the harmful effects of a poor diet through a new mechanism. It’s a new way of thinking about how the microbiome influences our health.”
“This shows how nutrition and our gut microbes can work together by producing molecules that fight inflammation and improve metabolic health,” added Professor Patrice Cani of Université catholique de Louvain and Imperial College London.
The research team included collaborators from Belgium, Canada, Australia, France, Italy and Spain. The work was supported by a range of national and international funders, including European and UK agencies such as the European Research Council and the Medical Research Council, as described in the study acknowledgments.
The authors note that TMA’s actions appear to differ from those of its liver‑derived co‑metabolite trimethylamine N‑oxide (TMAO), which has been associated in previous research with cardiovascular risk. In the context of diet‑induced obesity in mice, increasing TMA relative to TMAO by targeting the enzyme that converts TMA to TMAO improved immune tone and glucose control in their experiments, suggesting that carefully modulating this metabolic axis could be a future strategy to combat insulin resistance. However, the researchers stress that the current findings are based on preclinical models and mechanistic studies, and that more work will be needed before any clinical applications can be developed.