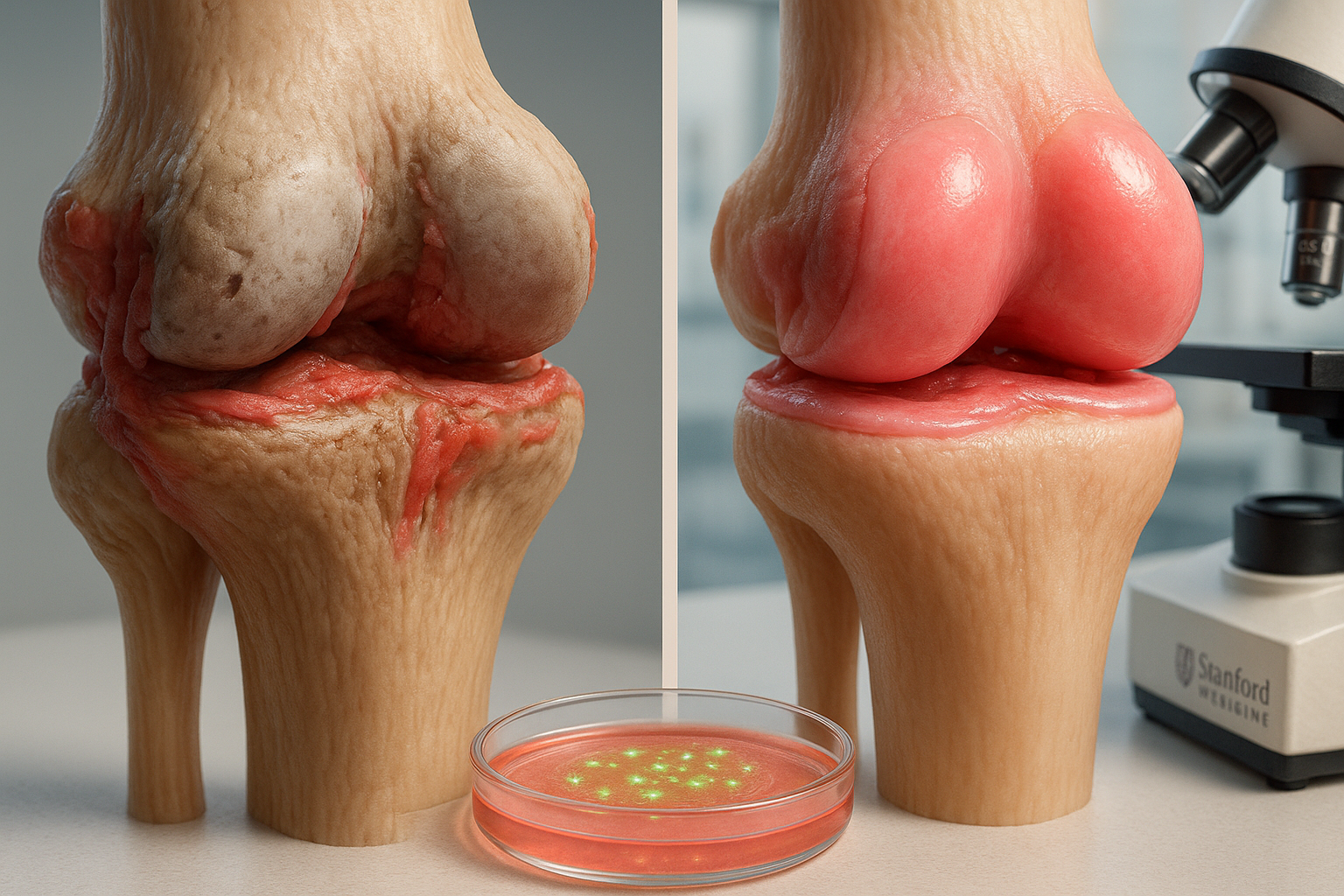
Stanford Medicine researchers report that blocking the enzyme 15-PGDH reversed age-related cartilage loss in older mice and reduced osteoarthritis-like damage after ACL-like knee injuries. In lab experiments, cartilage taken from knee replacement surgeries also showed early signs of regeneration after exposure to the inhibitor, findings published in *Science*.
Osteoarthritis affects about one in five adults in the United States and is associated with an estimated $65 billion a year in direct health care costs, according to Stanford Medicine’s summary of the work. Current care largely focuses on symptom relief or surgical joint replacement, and there are no approved drugs that can slow or reverse the underlying cartilage damage.
In a study published in Science, Stanford Medicine scientists focused on 15-hydroxy prostaglandin dehydrogenase (15-PGDH), an enzyme they describe as a “gerozyme” because its levels rise with age. The team reported that 15-PGDH levels increased in the articular cartilage of aged mice and in mice with joint injuries, and that inhibiting the enzyme altered cartilage cell behavior in ways consistent with repair.
In older mice, the researchers found that treatment with a small-molecule 15-PGDH inhibitor—given either systemically or injected into the knee—thickened cartilage across the joint surface. Tests indicated the new tissue was hyaline (articular) cartilage, rather than fibrocartilage, which is typically less functional.
The same inhibitor also reduced osteoarthritis development in a mouse model of knee injury resembling an ACL tear. The study reported that mice treated twice weekly for four weeks after injury were less likely to develop osteoarthritis and showed better movement and weight-bearing on the injured limb than untreated animals.
The researchers also tested human cartilage taken from patients undergoing total knee replacement for osteoarthritis. After one week of exposure to the inhibitor in the lab, the tissue showed fewer 15-PGDH–producing cells, lower expression of genes associated with cartilage breakdown and fibrocartilage, and early signs consistent with articular cartilage regeneration.
The study suggests cartilage repair occurred without activating stem cells. Instead, analyses indicated that existing cartilage cells (chondrocytes) shifted their gene-expression programs toward a more youthful, matrix-producing state.
“This is a new way of regenerating adult tissue, and it has significant clinical promise for treating arthritis due to aging or injury,” senior author Helen Blau, a Stanford professor of microbiology and immunology, said in Stanford Medicine’s release. Co-senior author Nidhi Bhutani, a Stanford associate professor of orthopaedic surgery, said the inhibitor “causes a dramatic regeneration of cartilage.”
The researchers noted that an oral 15-PGDH inhibitor is already being evaluated in Phase 1 clinical testing aimed at age-related muscle weakness, and they said they hope similar human trials will be launched to test cartilage regeneration.