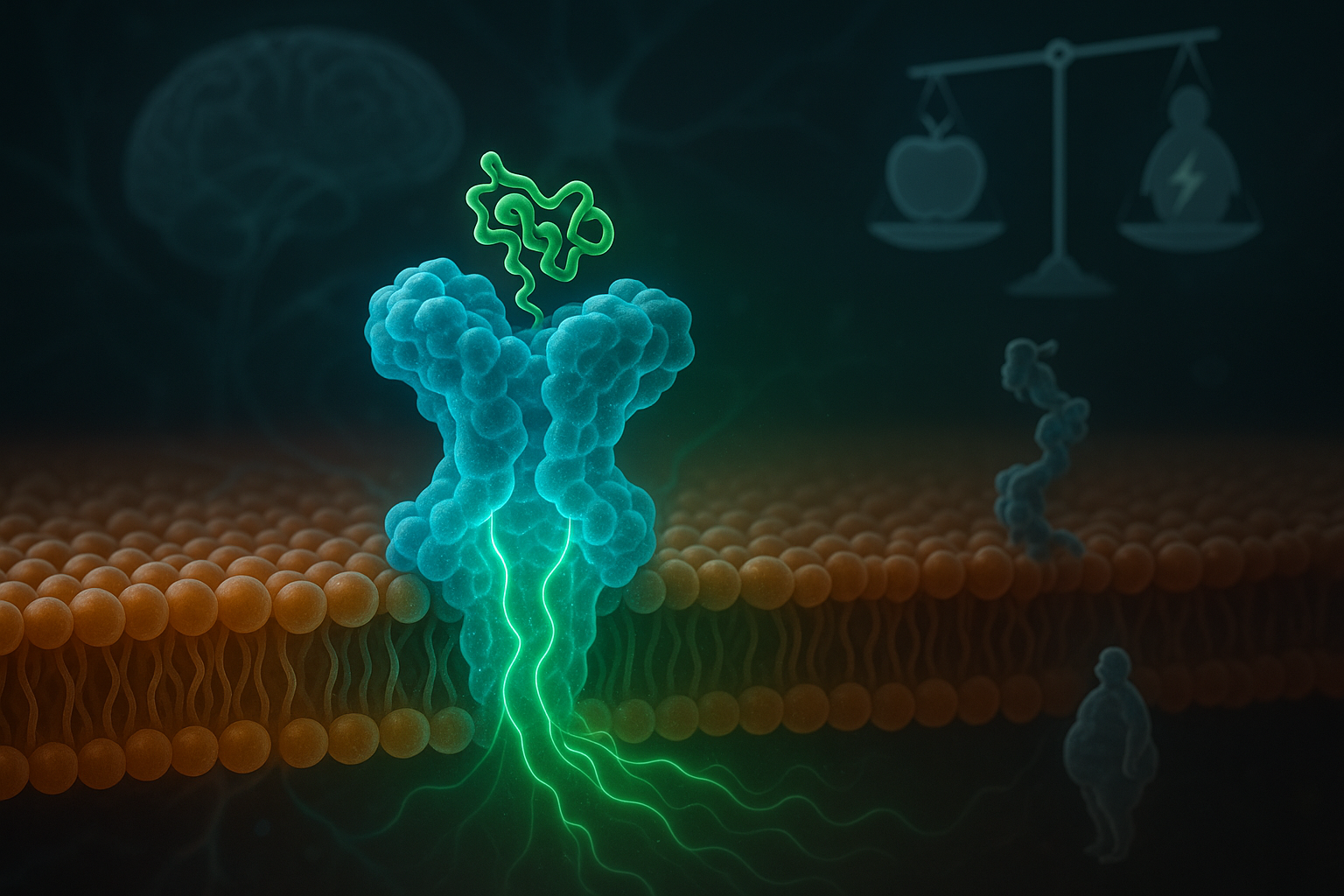
Researchers have shown that a helper protein called MRAP2 is crucial for the function of an appetite‑related receptor known as MC3R. The study, led by the University of Birmingham and published in Science Signaling, helps explain how genetic mutations in MRAP2 found in some people with obesity can weaken cellular signaling involved in energy balance, offering clues for future treatments.
A new study details the role of MRAP2, a small accessory protein, in regulating how the body handles hunger and energy. According to the University of Birmingham summary of the work, published in Science Signaling on December 16, an international research team showed that MRAP2 directly supports MC3R (melanocortin‑3 receptor), a receptor involved in energy homeostasis.
Using human hypothalamus transcriptomic data and cell‑based models, the scientists demonstrated that MRAP2 and MC3R are co‑expressed in neurons linked to energy balance and appetite control, and that MRAP2 physically interacts with MC3R. In HEK293 cell experiments, MRAP2 enhanced MC3R cyclic AMP (cAMP) signaling, impaired β‑arrestin recruitment, and reduced receptor internalization, helping sustain MC3R activity.
The team also examined how the relative amounts of the two proteins affect signaling. When MRAP2 was present at levels comparable to MC3R, MRAP2 boosted MC3R‑driven signaling, supporting the receptor’s role in balancing energy intake with energy use. Structural modeling, combined with alanine mutagenesis, identified specific transmembrane residues in MRAP2 and MC3R that are important for this regulatory effect.
Further experiments tested MRAP2 genetic variants previously identified in individuals who are overweight or obese. These altered forms of MRAP2 failed to enhance MC3R‑mediated signaling in the cell models, suggesting that such variants can disrupt hormone‑based pathways that normally help maintain energy balance and appetite control.
Dr. Caroline Gorvin, associate professor at the University of Birmingham and lead author, said in a statement released by the university: "The findings give us some important insights into what's going on in the hormonal system, related to some key functions like energy balance, appetite, and puberty timing.
"The identification of this protein, MRAP2, as a key aide or supporter to these essential appetite‑regulating proteins also gives us new clues for people who have a genetic predisposition to obesity, and how MRAP2 mutations are a clear indication of risk."
The work was carried out through the University of Birmingham’s Department of Metabolism and Systems Science and the Centre of Membrane Proteins and Receptors (COMPARE), a joint research centre involving the Universities of Birmingham and Nottingham that focuses on how cells communicate in health and disease.
By clarifying MRAP2’s role in MC3R signaling and highlighting the impact of obesity‑associated MRAP2 variants, the study points to potential targets for future drugs aimed at modulating appetite‑related pathways. Researchers hope such approaches could, in time, complement lifestyle interventions for managing weight and metabolic health.
The full study, titled "The accessory protein MRAP2 directly interacts with melanocortin‑3 receptor to enhance signaling," appears in Science Signaling (2025; 18(917)), DOI: 10.1126/scisignal.adu4315.