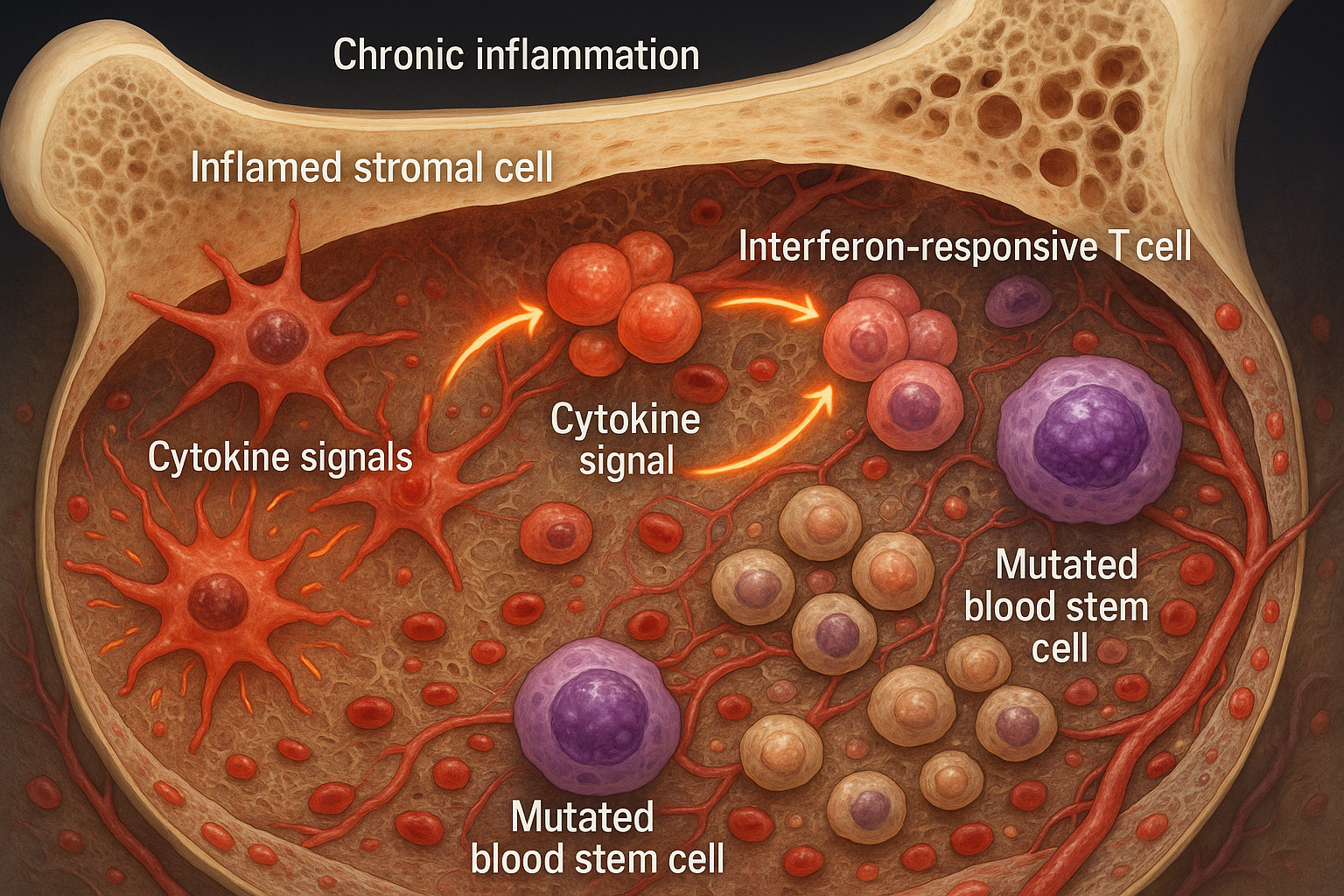
Chronic inflammation reshapes the bone marrow niche, fostering the expansion of mutated blood stem cells seen in clonal hematopoiesis and early myelodysplasia. The work, published November 18, 2025 in Nature Communications, maps a feed‑forward loop between inflammatory stromal cells and interferon‑responsive T cells and points to therapies that target the microenvironment as well as mutant cells.
What the study found
An international team co-led by Judith Zaugg (EMBL/University of Basel) and Borhane Guezguez (UMC Mainz) analyzed human bone marrow from individuals with clonal hematopoiesis of indeterminate potential (CHIP) and patients with myelodysplastic syndrome (MDS). They report that the marrow microenvironment becomes inflamed early, with inflammatory mesenchymal stromal cells (iMSCs) and interferon‑responsive T cells forming a circuit that undermines healthy blood production. The study was published November 18, 2025, in Nature Communications. (dx.doi.org)
According to EMBL, CHIP occurs in roughly 10–20% of adults over 60 and nearly 30% over 80, and is associated with a tenfold higher risk of blood cancers and about a twofold increase in cardiovascular disease and premature death. MDS affects up to 20 in 100,000 people over age 70, with around 30% of cases progressing to acute myeloid leukemia. (embl.org)
How they did it
Using single‑cell RNA sequencing, imaging of biopsies, proteomics, and co‑culture models, the team mapped changes across stromal, hematopoietic, and T‑cell compartments. They observed that iMSCs gradually replace supportive mesenchymal stromal cells and secrete interferon‑induced cytokines that attract and activate T cells, sustaining inflammation. (sciencedaily.com)
The researchers separated mutated from non‑mutated cells with SpliceUp, a computational method, and found that MDS stem cells failed to induce CXCL12 production by stromal cells—a signal important for homing and support of blood cells. (sciencedaily.com)
Important nuance
Press materials emphasize that the team did not see a direct inflammatory effect attributable to mutant hematopoietic cells in their analyses. The Nature Communications paper adds disease‑stage context: while healthy aged and CHIP HSPCs activated stromal support and MDS HSPCs failed to do so, MDS blasts further suppressed support and triggered inflammation—pointing to microenvironmental remodeling that precedes, and then intensifies with, disease progression. (sciencedaily.com)
Why it matters
The findings position the bone marrow niche—not just mutant cells—as a therapeutic target. The authors and institutional summaries suggest that anti‑inflammatory approaches or modulation of interferon signaling could help preserve marrow function or slow transitions from CHIP to MDS or AML; molecular signatures of iMSCs and interferon‑responsive T cells may serve as early biomarkers of risk. (sciencedaily.com)
A complementary Nature Communications study led by Marc Raaijmakers (Erasmus MC) reported an inflammatory T‑cell–stromal axis in MDS, reinforcing the idea that immune–stromal interactions drive early marrow failure and clonal evolution. (embl.org)