Researchers report that a tetrapeptide called CAQK, delivered intravenously after traumatic brain injury, accumulated in damaged brain regions and was associated with reduced lesion size and inflammatory signals in mice, with similar targeting seen in pigs. The team and a related startup say they plan to seek U.S. FDA clearance to begin early-stage human testing, though no timeline has been announced.
A team of researchers spanning academia and a startup company has reported preclinical results for a potential traumatic brain injury (TBI) therapy built around an extremely short peptide, CAQK.
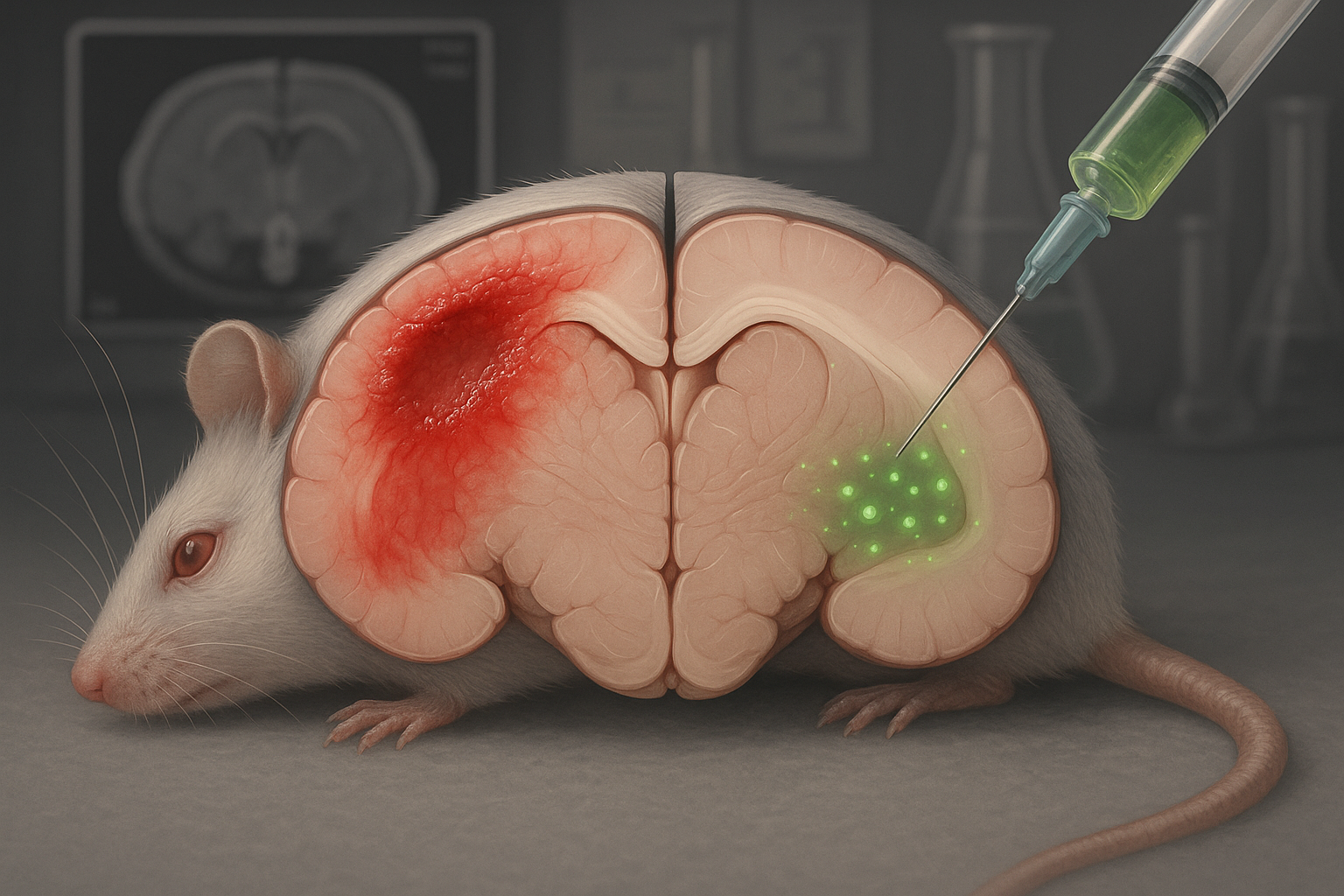
In a study published in EMBO Molecular Medicine in October 2025, the authors describe CAQK as a neuroprotective “tetrapeptide” (a four–amino-acid peptide) that can be administered intravenously soon after moderate-to-severe brain injury and that preferentially accumulates in injured brain tissue in animal models.
TBI commonly follows blows to the head in events such as traffic crashes, workplace accidents and falls. The researchers cite an incidence estimate of about 200 cases per 100,000 people per year. They also note that acute clinical care primarily focuses on stabilizing patients—such as controlling intracranial pressure and maintaining cerebral blood flow—and that there are currently no approved drugs that directly stop the initial injury-related damage or the subsequent cascade that can include inflammation and cell death.
The new work builds on earlier research published in 2016 that identified CAQK as a peptide that “homes” to acute brain injury sites after systemic administration. In that earlier study, CAQK was described as binding targets in the extracellular matrix that are upregulated after injury and was investigated as a means to deliver imaging agents or therapeutic payloads to damaged brain tissue.
In the 2025 study, the authors tested CAQK itself as a therapy. After intravenous dosing shortly after injury, the peptide accumulated in damaged regions of the brain in both mice and pigs. The team reports that CAQK binds glycoprotein- and proteoglycan-rich components of the extracellular matrix that increase after injury.
In mouse experiments, animals treated with CAQK had smaller lesions than controls, along with measures consistent with reduced cell death and lower expression of inflammatory markers in injured tissue. The researchers also report improvements on behavioral and memory tests after treatment and state that they observed no evident toxicity under the study conditions.
The study’s first author, Aman P. Mann, said the team saw “less cell death and lower expression of inflammatory markers” in the injured area and reported improved functional testing outcomes “with no evident toxicity.” Co-author Pablo Scodeller said the peptide’s simplicity and manufacturability—along with attributes the researchers describe as favorable for tissue penetration and low immunogenicity—make it a promising candidate for further development.
According to a ScienceDaily report based on materials from the Spanish National Research Council (CSIC), the startup AivoCode—linked to study authors—plans to seek permission from the U.S. Food and Drug Administration to begin Phase I clinical trials in humans, though the company has not announced a timeline.
The findings remain preclinical, and the authors emphasize that additional studies would be needed to establish safety, dosing, and effectiveness in humans.