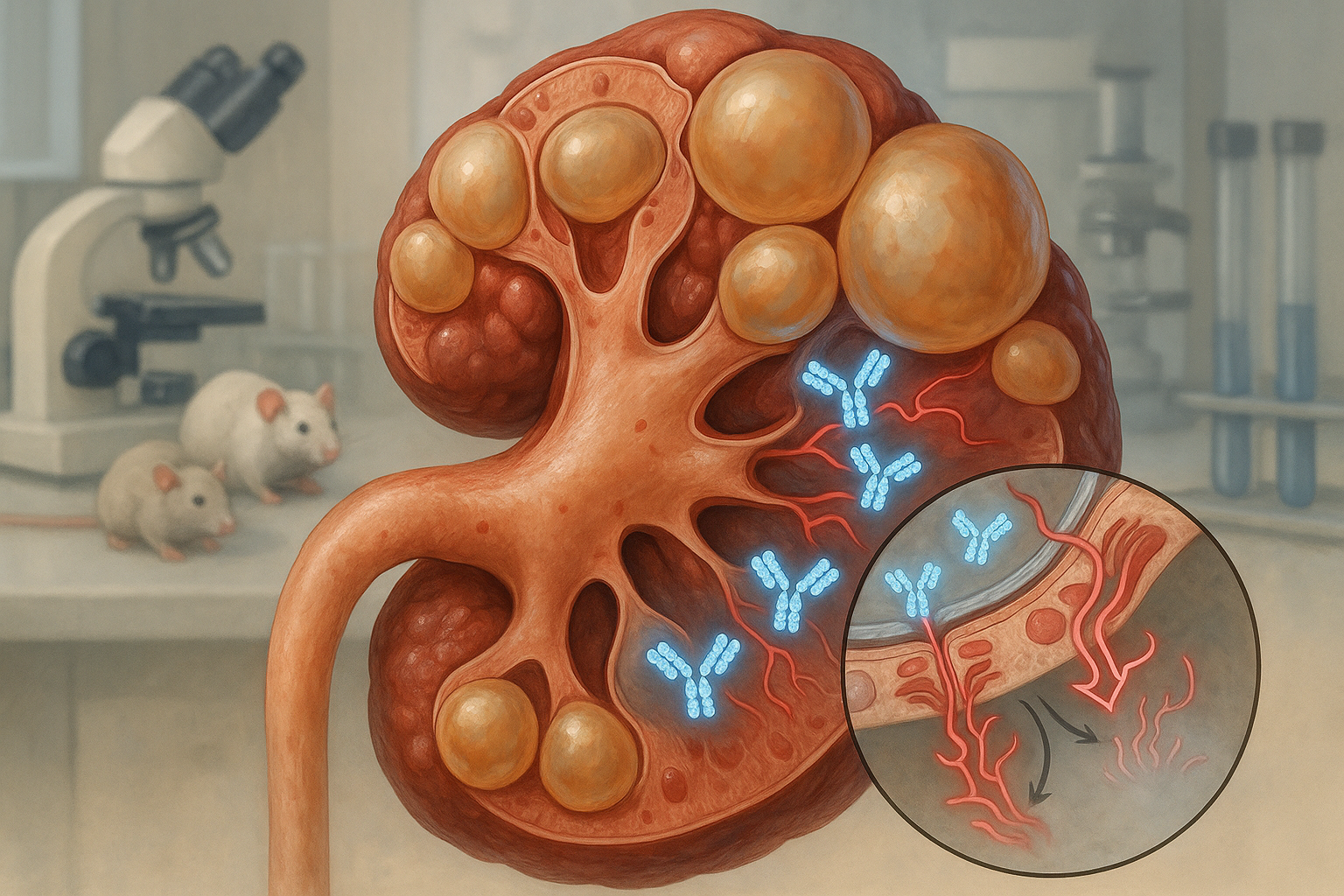
UC Santa Barbara researchers report a dimeric IgA monoclonal antibody that can cross cyst-lining epithelia and dampen cMET signaling in polycystic kidney disease. In rodent models, it accumulated inside cysts, reduced pathway activity and slowed disease without apparent harm to healthy tissue, according to the study and the university’s release.
Polycystic kidney disease is a genetic disorder in which fluid-filled cysts form in the kidneys, often damaging tissue over time and, in advanced cases, leading to dialysis. There is no cure. (niddk.nih.gov)
A UC Santa Barbara team led by biologist Thomas Weimbs describes a monoclonal antibody engineered in dimeric immunoglobulin A (dIgA) format to reach the interiors of kidney cysts and block growth signaling via the cMET receptor. The work, published in Cell Reports Medicine and led by first author Margaret F. Schimmel, details preclinical tests in animal models. (news.ucsb.edu)
“The cysts just keep growing endlessly,” Weimbs said. “And we want to stop them. So we need to get a drug into these cysts that will make them stop.” (news.ucsb.edu)
Existing small‑molecule options can slow cyst growth but carry side effects. Conventional IgG antibodies—highly successful in oncology—typically do not traverse the cyst epithelium, limiting their usefulness in PKD. The UCSB team instead reformatted an IgG into a dIgA backbone to improve access to cyst interiors. (news.ucsb.edu)
That strategy builds on earlier findings that the polymeric immunoglobulin receptor (pIgR), abundant on cyst‑lining cells, can actively transcytose dIgA from the bloodstream into cyst lumens. In mouse PKD models and human tissues, dIgA targeted cysts whereas IgG did not, supporting the rationale for dIgA‑based therapeutics. (pmc.ncbi.nlm.nih.gov)
In the new study, a dIgA antibody against cMET localized inside cysts in mouse and rat models, reduced cMET activity and slowed disease progression without detected adverse effects, the authors report. The university’s summary also notes a “dramatic onset of apoptosis” in cyst epithelial cells—but not in healthy renal tissue—following treatment. (pmc.ncbi.nlm.nih.gov)
The research is preclinical. Funding cited for the work includes grants from the National Institutes of Health and the U.S. Department of Defense. Co‑authors listed in the paper are Bryan C. Bourgeois, Alison K. Spindt, Sage A. Patel, Tiffany Chin, Gavin E. Cornick, Yuqi Liu and Weimbs. (pmc.ncbi.nlm.nih.gov)
Looking ahead, Weimbs said the team aims to compare antibodies against multiple growth factors and receptors found in cyst fluid—and potentially combine them—to identify the most effective strategies. Partnerships and manufacturing access will be needed to produce and test additional candidates. “It would be a good idea to compare blocking of several different growth factors and several receptors, maybe side‑by‑side … That would be the next step,” he said. (news.ucsb.edu)