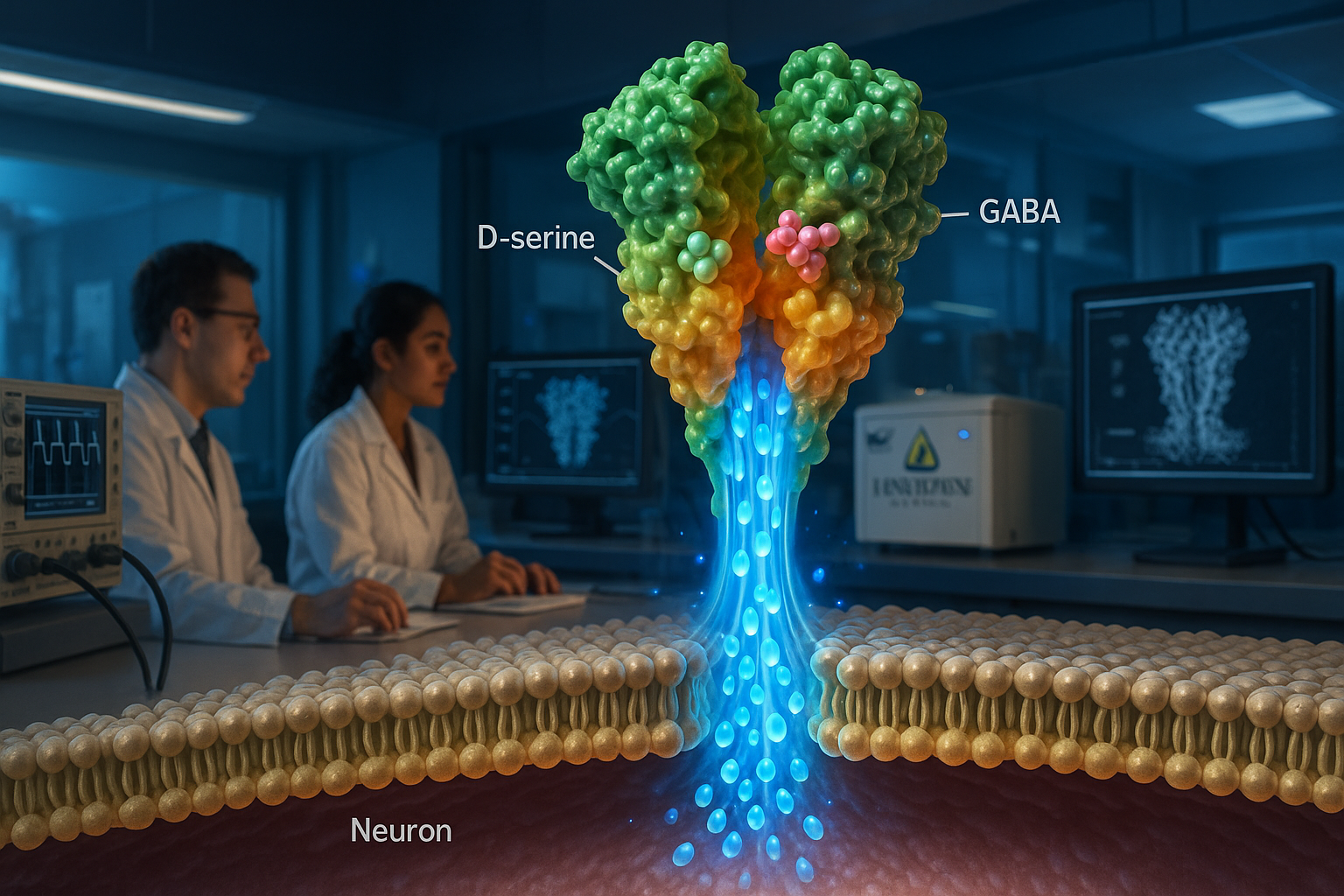
Johns Hopkins Medicine researchers report that delta-type ionotropic glutamate receptors (GluDs)—long debated as to whether they conduct ions—can act as ligand-gated ion channels. The Nature study used cryo-electron microscopy and membrane recording experiments to characterize human GluD2 and found it can be activated by the neurotransmitters D-serine and GABA, findings the authors say could help guide drug development for disorders linked to GluD mutations.
Researchers at Johns Hopkins Medicine say they have clarified the long-standing question of whether delta-type ionotropic glutamate receptors, known as GluDs, can function as ion channels.
In a paper published in Nature, the team reports that purified human GluD2 (hGluD2) behaves as a ligand-gated ion channel in vitro. The researchers used cryo-electron microscopy alongside membrane electrical recording experiments to characterize the receptor’s structure and channel activity.
“This class of protein has long been thought to be sitting dormant in the brain,” said Edward Twomey, Ph.D., an assistant professor of biophysics and biophysical chemistry at the Johns Hopkins University School of Medicine, according to a Johns Hopkins Medicine release carried by ScienceDaily.
The study reports that hGluD2 can be activated by the neurotransmitters D-serine and GABA, and that activation is stronger at physiological temperatures. The authors also describe how the receptor’s ligand-binding domains are coupled to an ion channel pore, providing a structural explanation for how binding can trigger channel opening.
The researchers also examined a cerebellar ataxia–linked point mutation in the ligand-binding domain and report that it alters receptor architecture and can produce so-called “leak” currents in their experiments.
Johns Hopkins Medicine said the findings may help inform efforts to design drugs that modulate GluD activity in disorders associated with GluD mutations, including psychiatric conditions such as anxiety and schizophrenia, and neurological disorders affecting movement.
The Nature article lists Haobo Wang, Fairine Ahmed, Jeffrey Khau, and Anish Kumar Mondal as co-authors with Twomey. ScienceDaily, citing Johns Hopkins Medicine materials, also reports that Johns Hopkins University has filed a patent covering techniques used to measure electrical currents from GluDs and that the work was funded by the National Institutes of Health, the Searle Scholars Program, and the Diana Helis Henry Medical Research Foundation.
The paper is titled “Delta-type glutamate receptors are ligand-gated ion channels” and appears in Nature (volume 647, issue 8091, pages 1063–1071; published online Sept. 16, 2025; issue date Nov. 27, 2025).